发布时间:2022-09-26 10:32:47发布者:万孚卡蒂斯
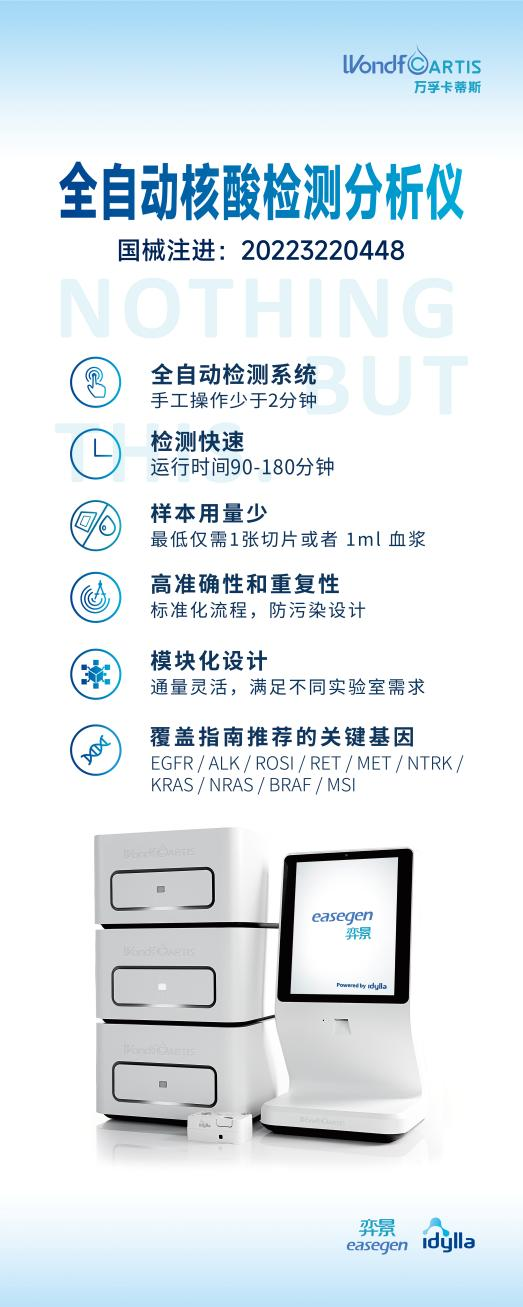
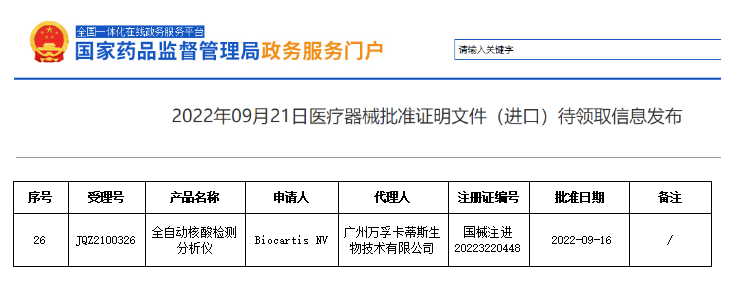
2022年9月16日,万孚卡蒂斯“全自动核酸检测分析仪”正式获批国家药品监督管理局(NMPA)三类医疗器械认证,注册证号为:国械注进20223220448.
On September 16th, 2022,A Idylla™ (Mainland China trademark, easegene™)fully automatic molecular diagnostic platform was officially approved by the NMPA of China as a category III medical device with the registration certificate number:国械注进20223220448.
关于Idylla™(弈景®)
全自动分子诊断平台Idylla™(中国商标品牌为“弈景®)开发用于指导肺癌、结直肠癌和黑色素瘤等治疗的10多个伴随诊断产品,致力于为肿瘤患者提供精确、灵敏、便捷的诊断及用药指导!
弈景®,让时间不是距离,让空间不成阻隔。这是一款基于实时荧光PCR技术的弈景全自动化核酸检测分析系统,整合了从样本处理到结果分析的全过程,全程仅需约2小时,及时为治疗决策提供诊断依据;卓越的易用性和防污染设计,克服了传统分子诊断的障碍和壁垒,可用于各类型的实验室,更加贴近患者。
About Idylla™ (Mainland China trademark, easegene™)
The fully automatic molecular diagnostic platform - Idylla™ (Mainland China trademark, easegene™)was launched to guidethe treatment of lung cancer, colorectal cancer, melanoma and etc. for over 10 companion diagnostics products. This platform is committed to providing accurate, sensitive and fast diagnosis for medicine guidance.
easegene™shorten the distance of the time and break the barrier of the space.easegene™, a fully automated, sample-to-result PCR-based molecular diagnostic system, integrates the whole process from sample processing to result analysis, which only takes about 2 hours, and provides diagnostic basis for treatment decision-making in time. The superior ease of use and anti-contamination design overcomes theobstacles and barriers of traditional molecular diagnostics and can be used in all types of laboratories, closer to the patient.